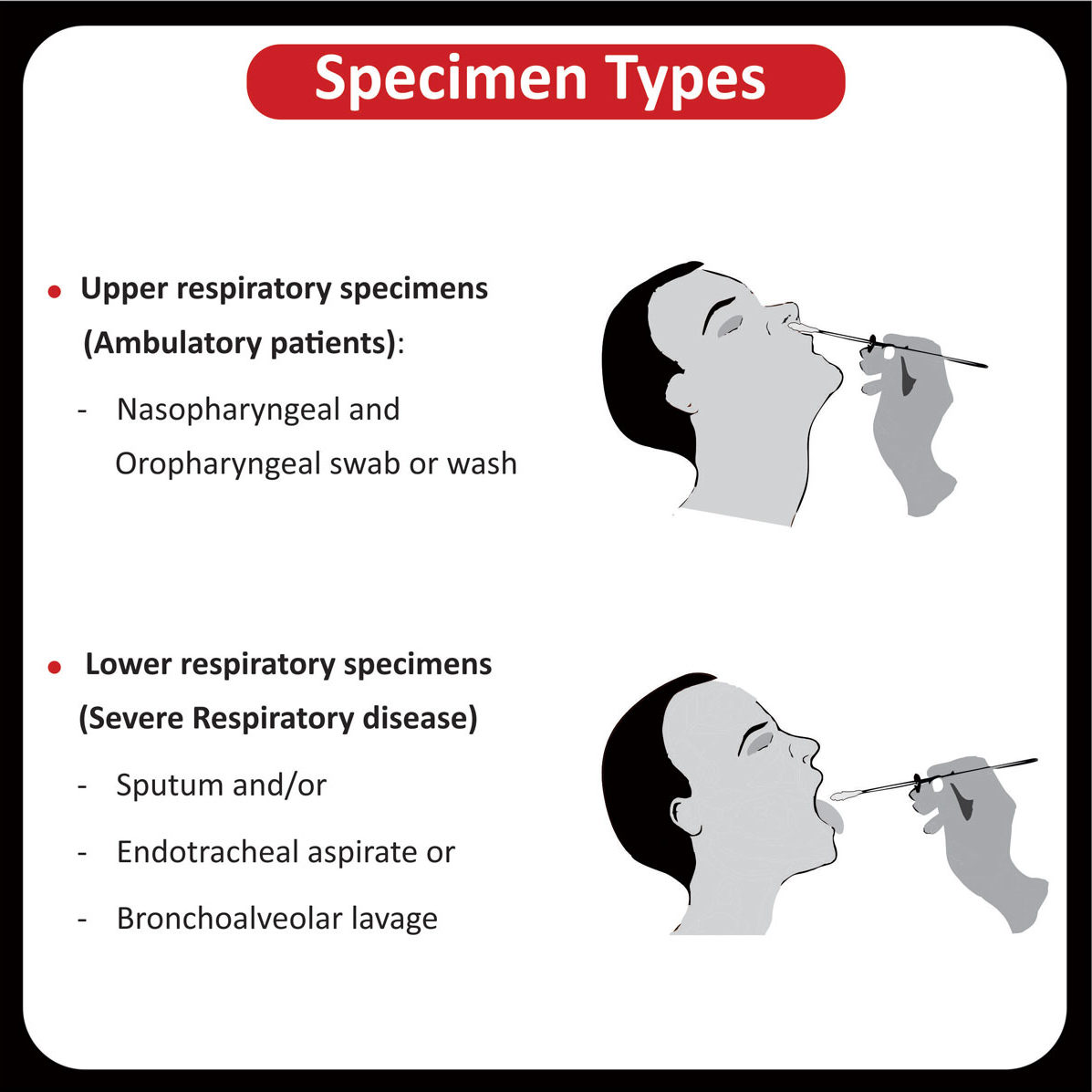
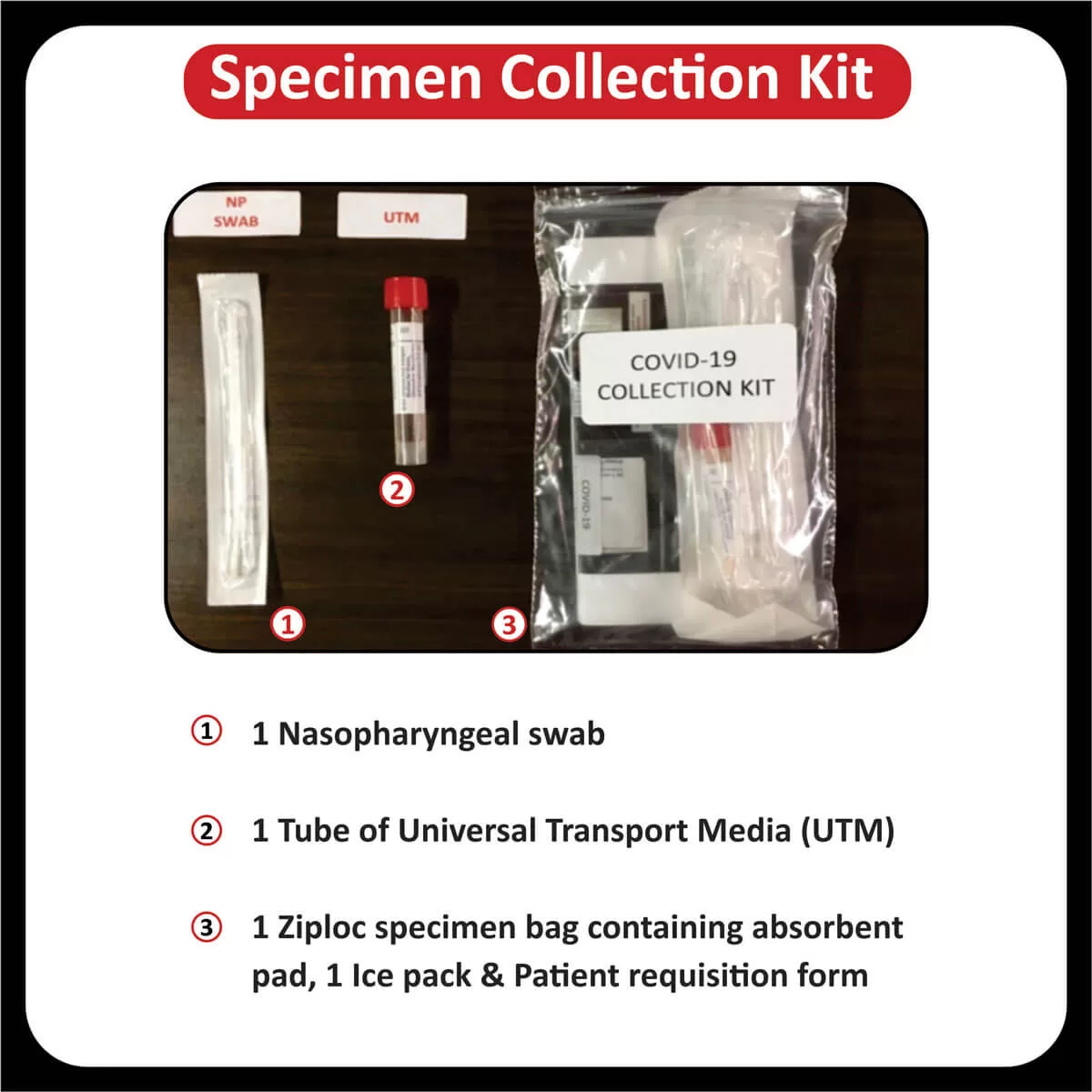
In a recent clinical trial, researchers found that patients with a higher tumor mutational burden, T cell inflamed gene expression profile and PDL-1 expression showed a better response rate and progression-free survival across different cancers (Read more).
In another clinical trial, researchers found that adjuvant modified FOLFIRINOX (fluorouracil, leucovorin, irinotecan, oxaliplatin) produced better disease-free and overall survival than gemcitabine in patients with resected pancreatic cancer (Read more).
The FDA approved Cabozantinib for patients with hepatocellular carcinoma (HCC) who have been previously treated with Sorafenib. Overall survival was improved by 2.2 months with Cabozantinibversus placebo representing a 24% reduction in risk of death (Read more).
The FDA approved Ibrutinib in combination with Obinutuzumab in treatment of naïve patients with Chronic Lymphocytic leukemia/Small Lymphocytic Lymphoma (Read more). This is the first approval of a non-chemotherapy combination regimen for treatment of naive CLL/SLL patients.
The FDA approved Trastuzumab-dttb (Ontruzant), a biosimilar referencing Trastuzumab across all eligible indications including HER2 overexpressing breast cancer, metastatic breast cancer, metastatic gastric cancer and gastroesophageal junction adenocarcinoma in patients who have not received prior treatment for metastatic disease (Read more). This is a third Trastuzumab biosimilar approved by the FDA.
As per latest trial results, Pembrolizumab significantly improved overall survival in PDL-1 expressing esophageal cancer patients with combined positive score (CPS) of 10 or greater compared to Docetaxel, Paclitaxel or Irinotecan (Read more).
In another clinical trial, renal cell carcinoma patients showed improved significant overall survival with Nivolumab plus Ipilimumab compared to Sunitinib (Read more).