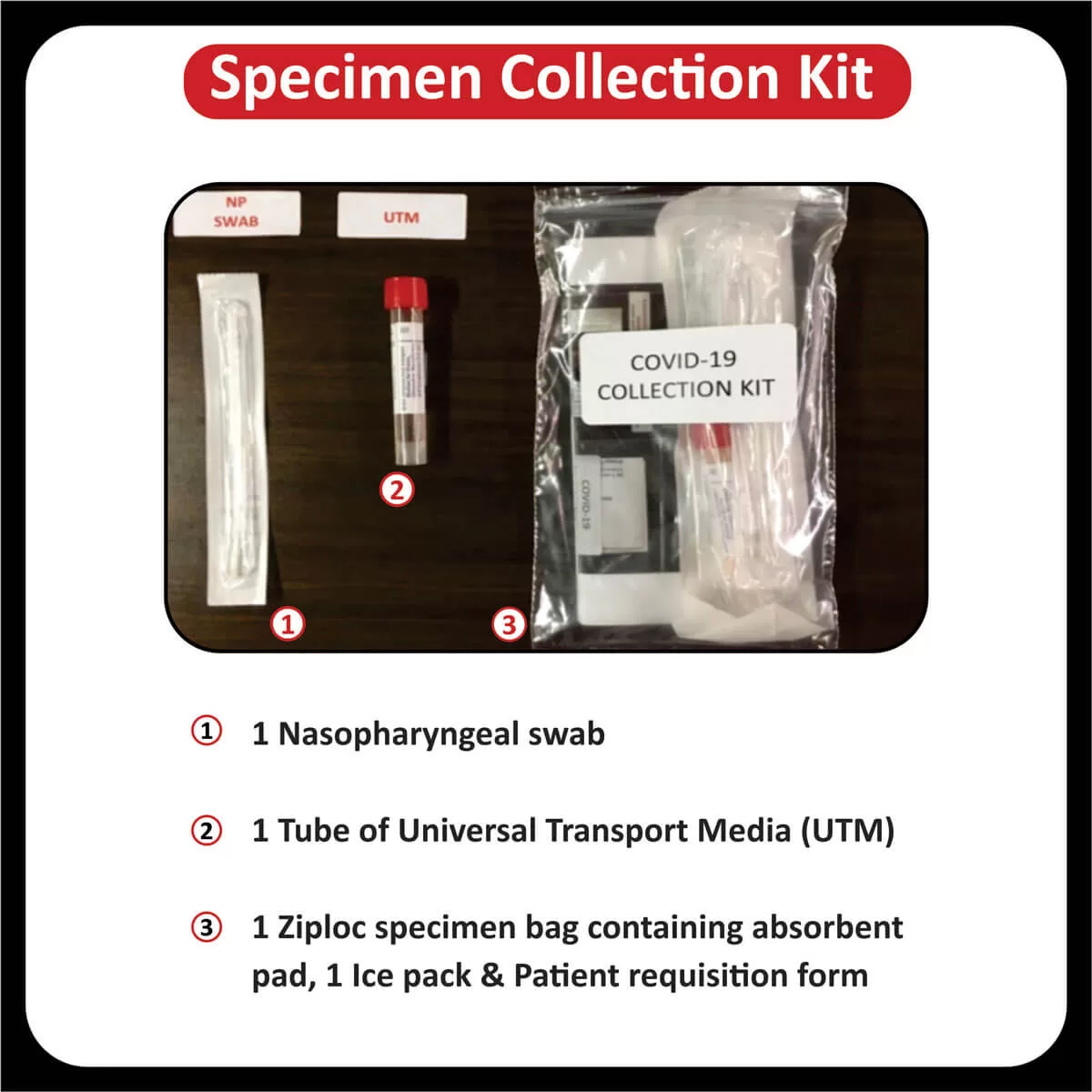
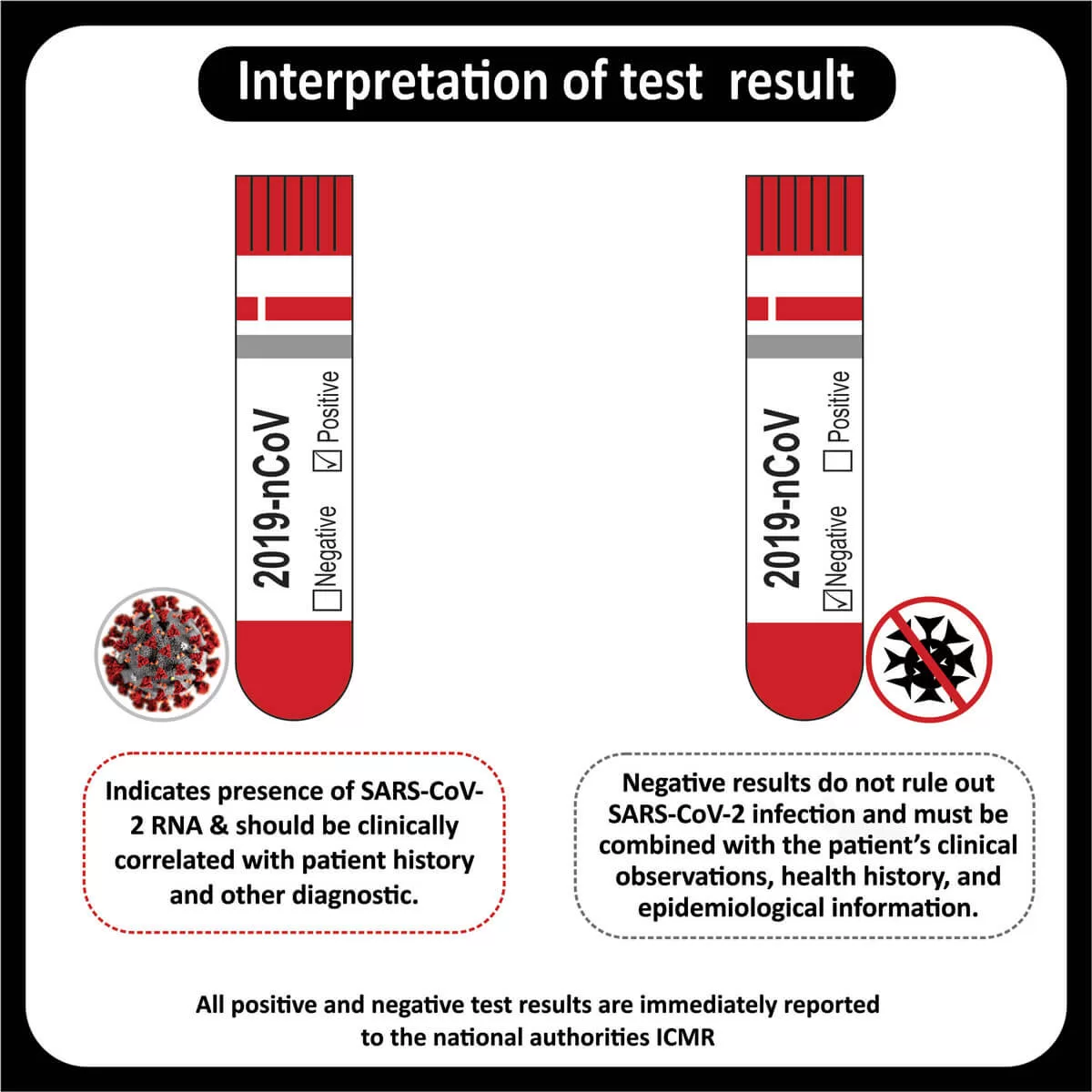
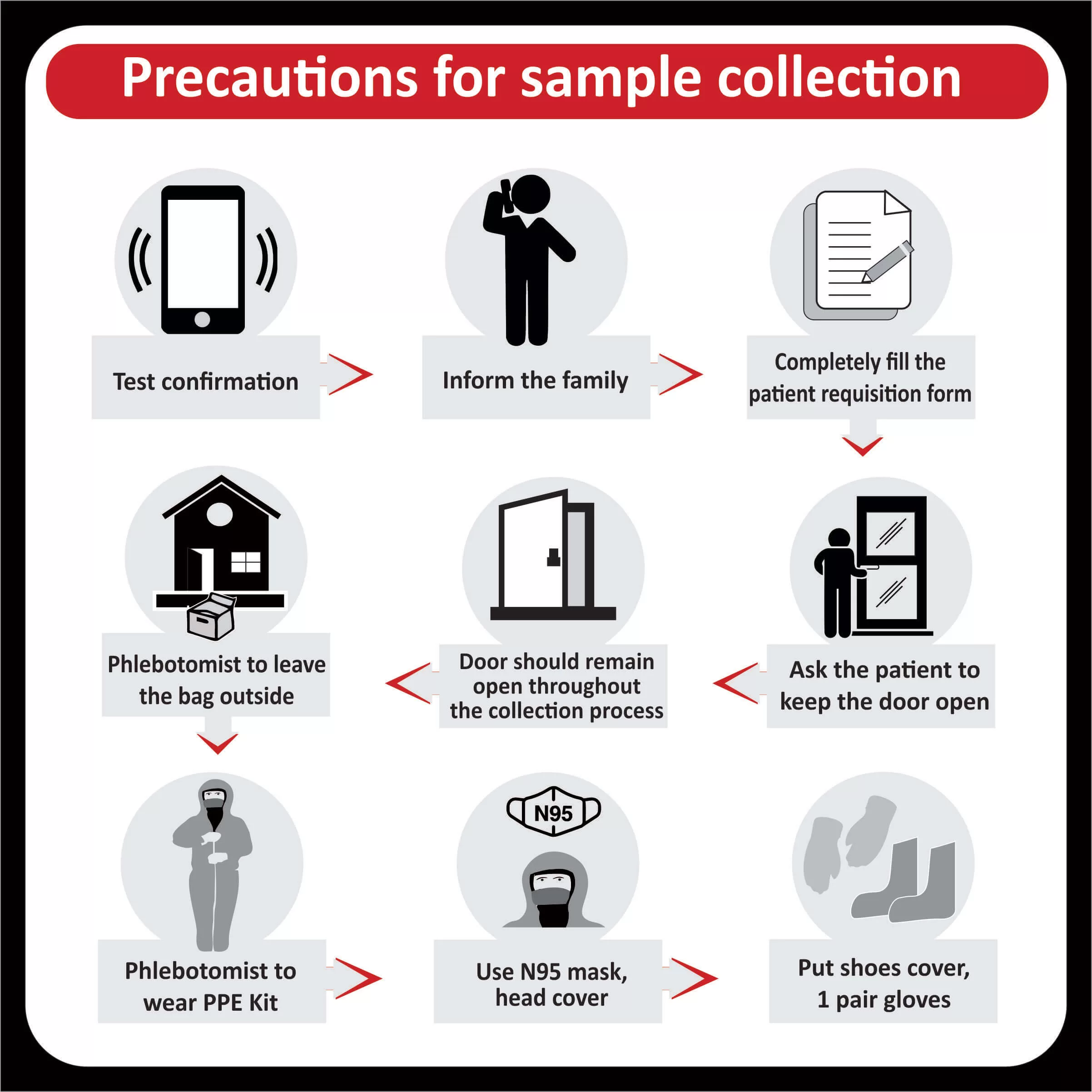
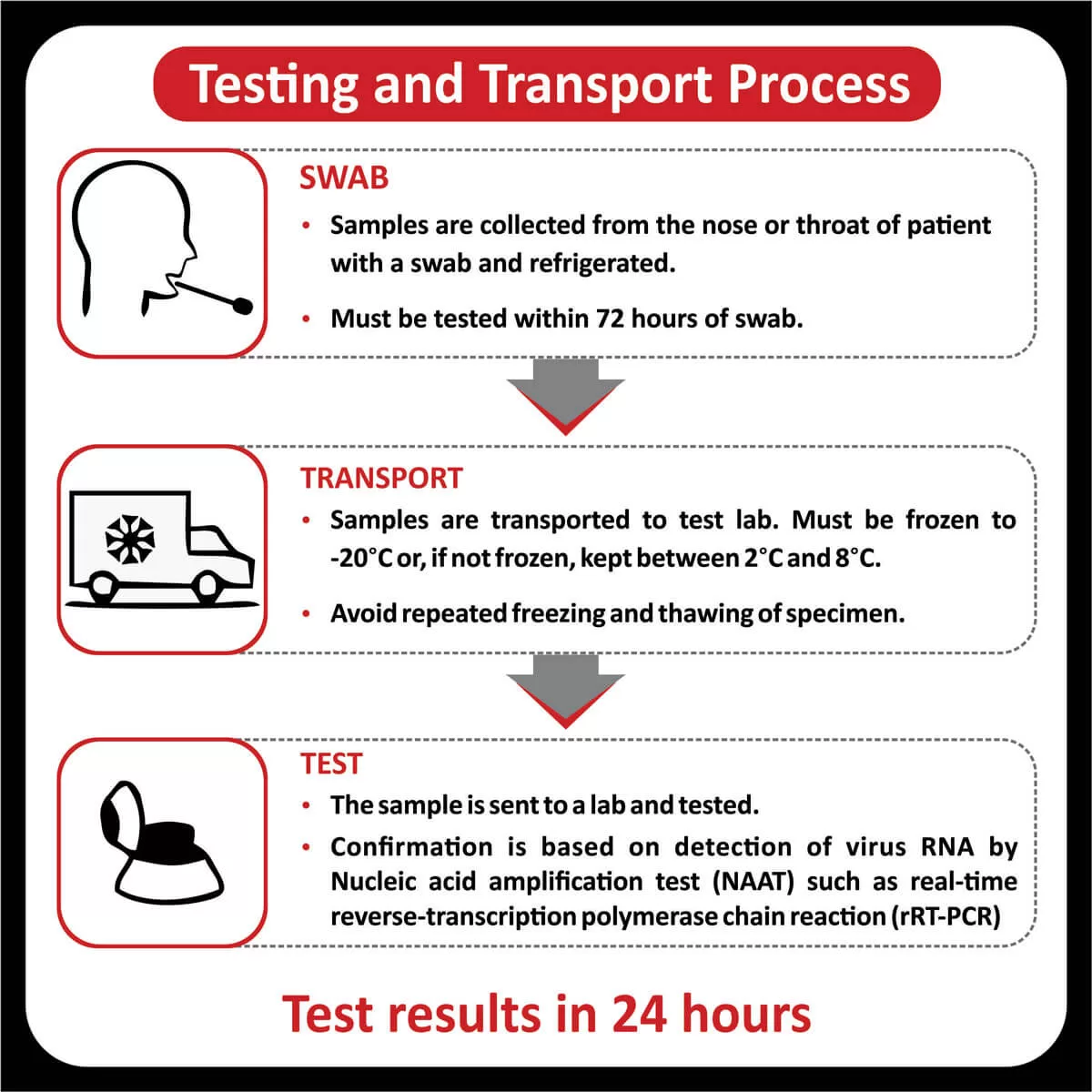
The FDA approved Osimertinib (Tagrisso) for the first-line treatment of metastatic NSCLC with (EGFR) exon 19 deletions or exon 21 L858R mutations (Read More).
The FDA approved Pembrolizumab in combination with chemotherapy (Pemetrexed and Platinum) for first-line treatment of metastatic nonsquamous NSCLC with no EGFR or ALK genomic tumor aberrations (Read more).
The FDA approved Roche Cobas EGFR mutation test as CDx for Iressa (Read more).
The FDA approved Lorlatinib for second or third-Line treatment of ALK-Positive metastatic NSCLC (Read more).
Nivolumab/Ipilimumab showed prolonged progression-free survival vs chemotherapy in patients with high tumor mutational burden (Read more).
Addition of Pembrolizumab to chemotherapy significantly improved overall and progression-free survival in patients with non-squamous non-small cell lung cancer (Read more).
Women with advanced triple-negative breast cancer and a BRCA mutation were twice as likely to benefit from Carboplatin in comparison to Docetaxel – the current standard of care (Read more).
Addition of Everolimus to Fulvestrant (Faslodex) improved progression-free survival in post-menopausal womenwithHR positive, HER2-negative metastatic breast cancer (Read more).
The FDA expanded approval for Ribociclib indication in hormone receptor-positive, HER2-negativeadvanced or metastatic Breast cancer (Read more).
The FDA approved Pembrolizumab for patients with recurrent or metastatic cervical cancer whose tumors express PD-L1 (CPS ≥1) (Read more).
The FDA approved Venetoclax (Venclexta) for treatment of patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), with or without 17p deletion, following at least one prior therapy (Read more).
The FDA granted breakthrough therapy designation to Quizartinib, an investigational FLT3 inhibitor, for the treatment of adult patients with relapsed/refractory FLT3-ITD Acute myeloid leukemia (AML) (Read more).
The FDA granted approval to Gilteritinib for the treatment of adult patients who have relapsed or refractory acute myeloid leukemia (AML) with a FLT3 mutation (Read more).
The FDA granted accelerated approval to Pembrolizumab for advanced Gastric cancer (Read more).
The FDA approved Dako PD-L1 IHC 22C3 PharmDx Assay CDx to select patients with locally advanced or metastatic urothelial carcinoma who are not eligible for Cisplatin treatment with Keytruda (Read more).
The FDA approved Nivolumab + Ipilimumab for second-line treatment of MSI-H/dMMR metastatic Colorectal cancer (Read more).
The FDA granted breakthrough therapy designation to LOXO-292 for RET fusion-positive Thyroid cancer (Read more).