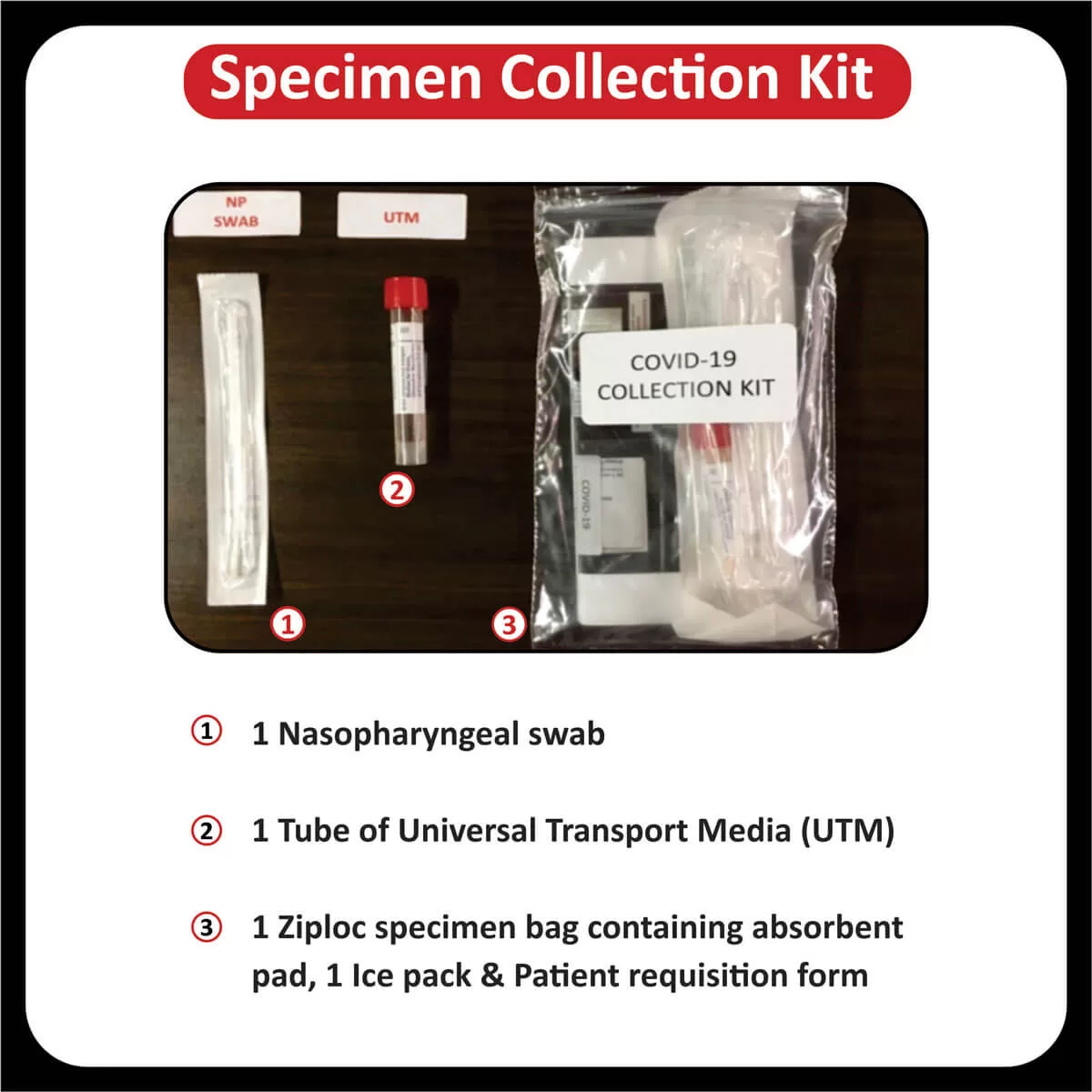
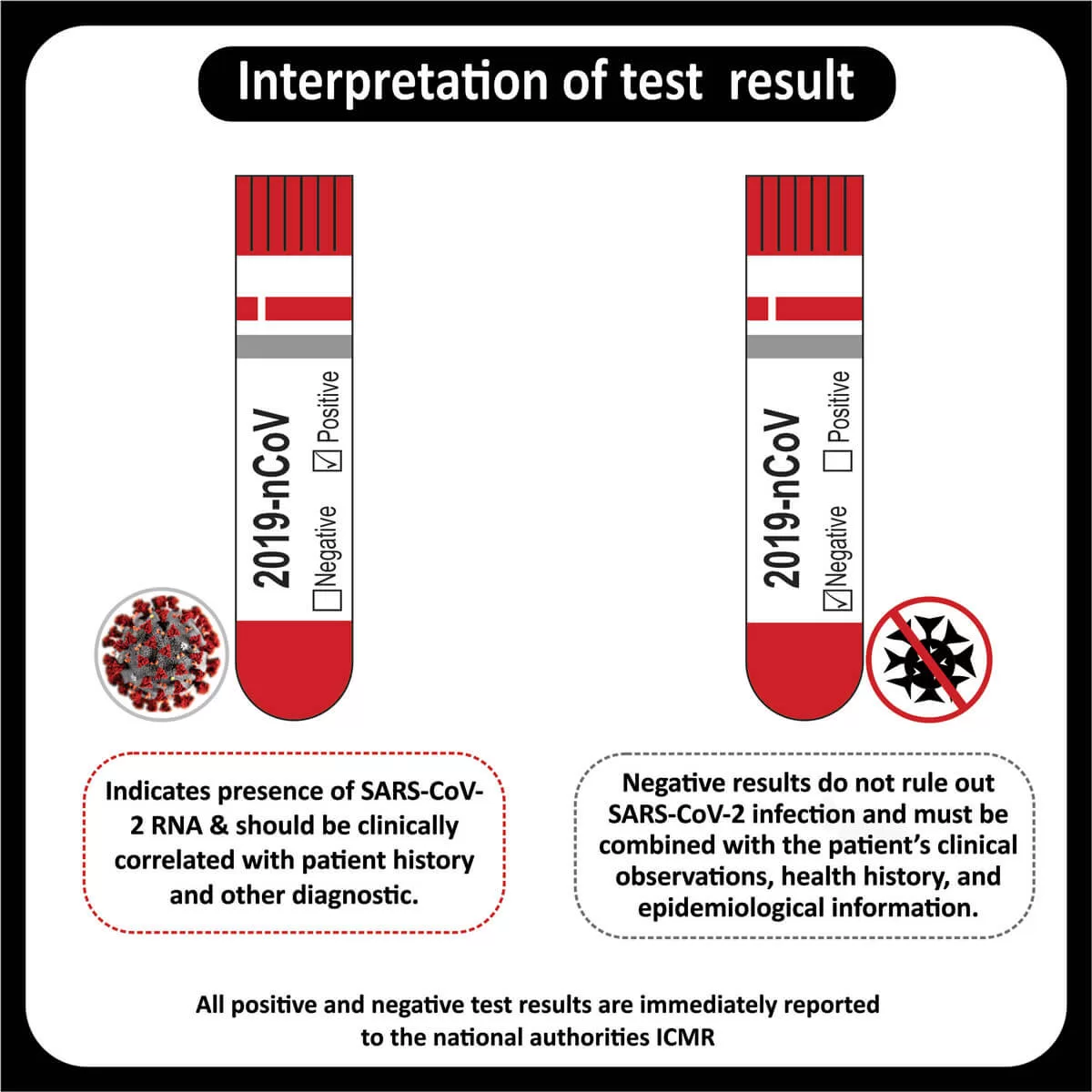
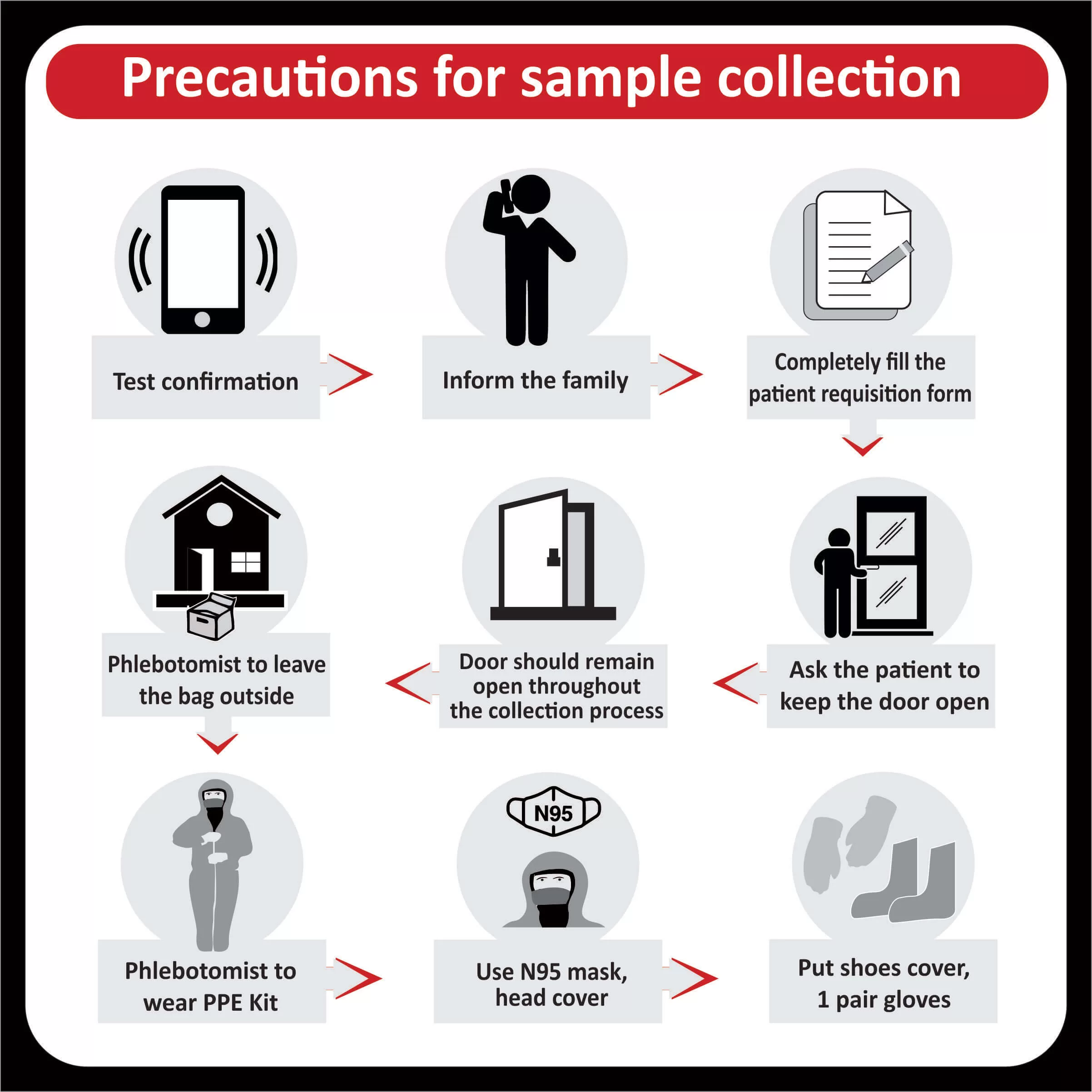
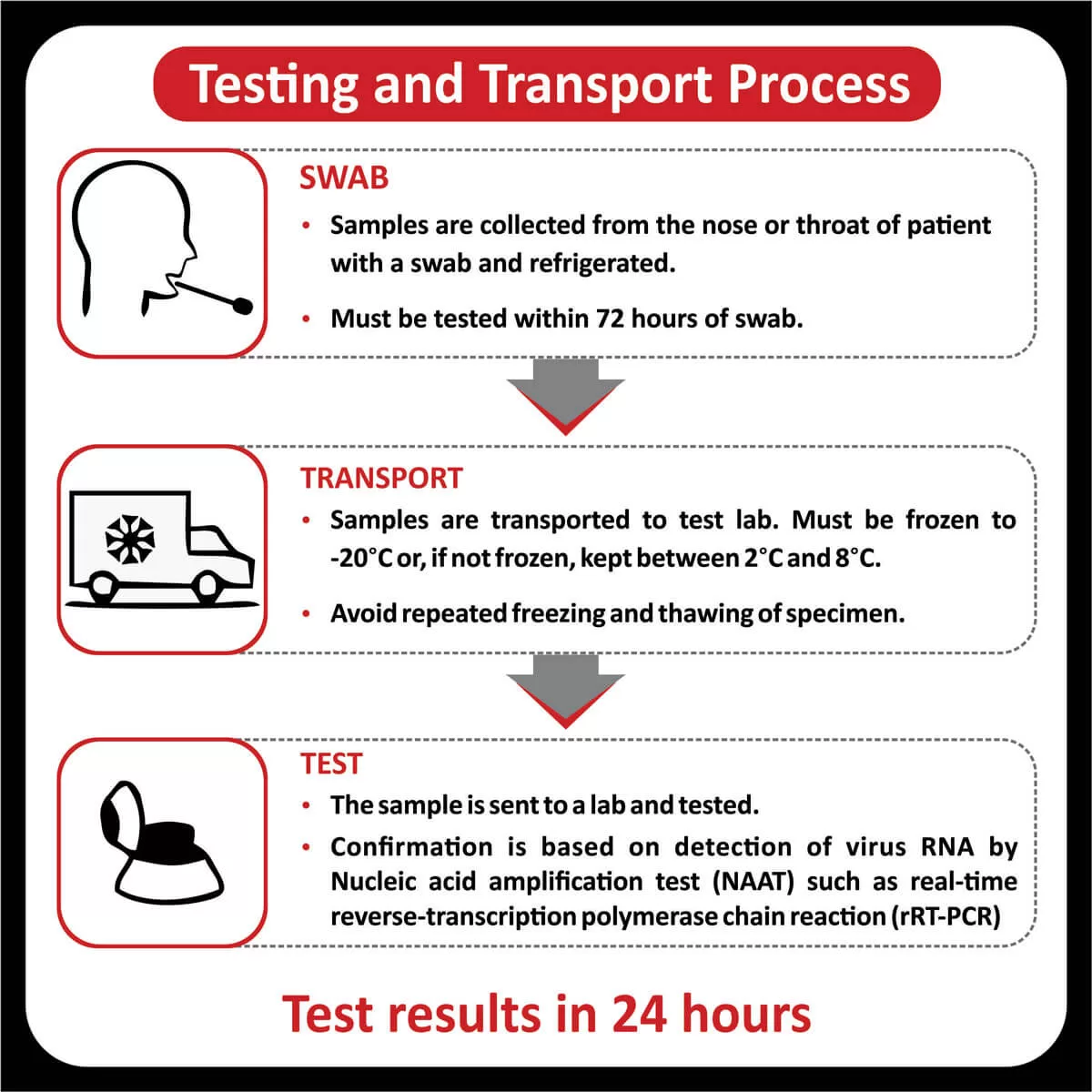
NEW YORK (GenomeWeb) – Precipio said today that India’s Core Diagnostics will now offer liquid biopsy testing using Precipio’s ICE COLD-PCR technology to major hospital networks throughout India and the surrounding region.
Financial terms of the laboratory services agreement were not disclosed.
Precipio promotes ICP, which it exclusively licensed from the Dana Farber Cancer Institute, as offering similar sensitivity to droplet-based digital PCR platforms using less DNA and for less cost. This makes the platform a good fit for the Indian market, which is “both clinically advanced and fiscally responsible, particularly when it comes to healthcare expenditures,” Precipio president and CEO Ilan Danieli said in a statement.