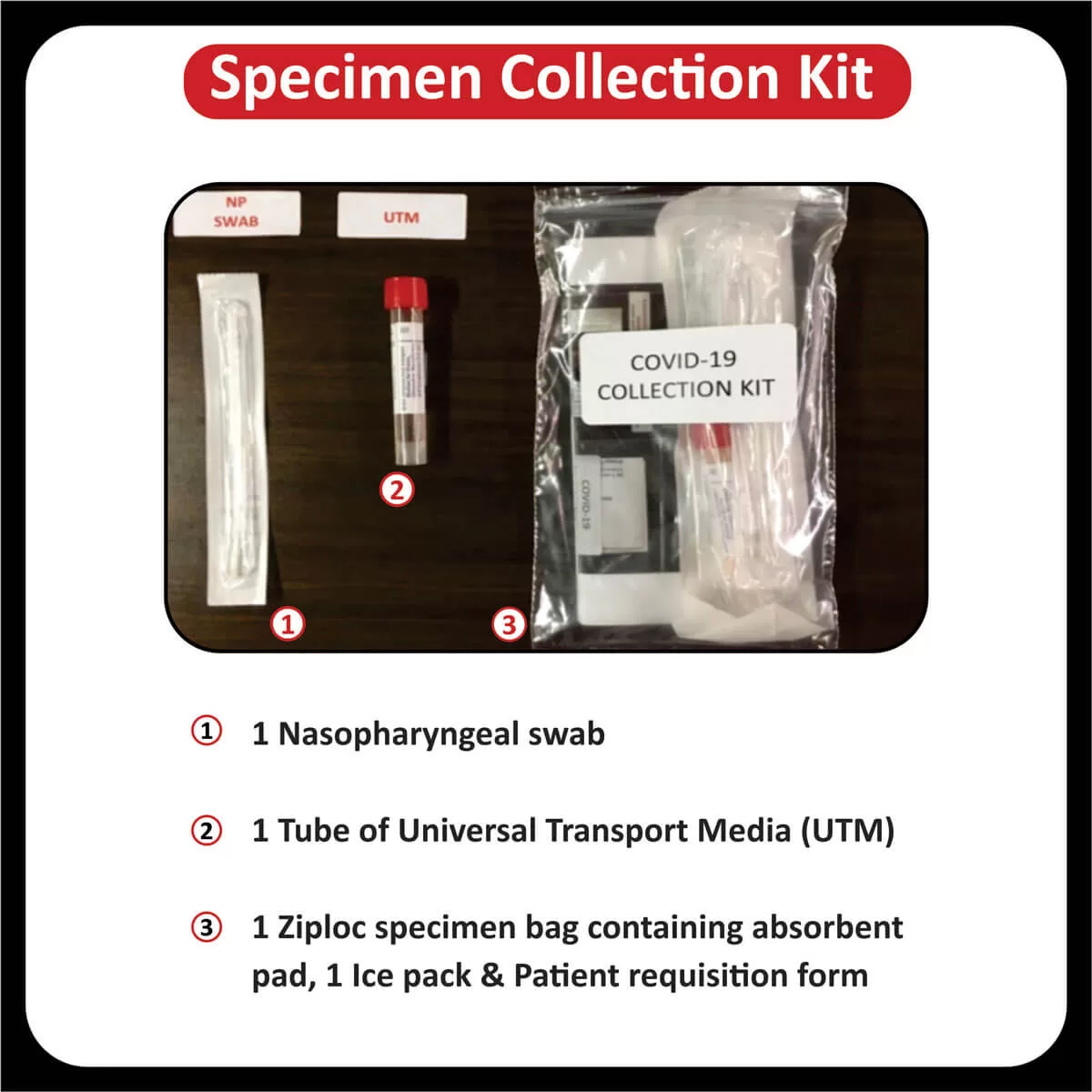
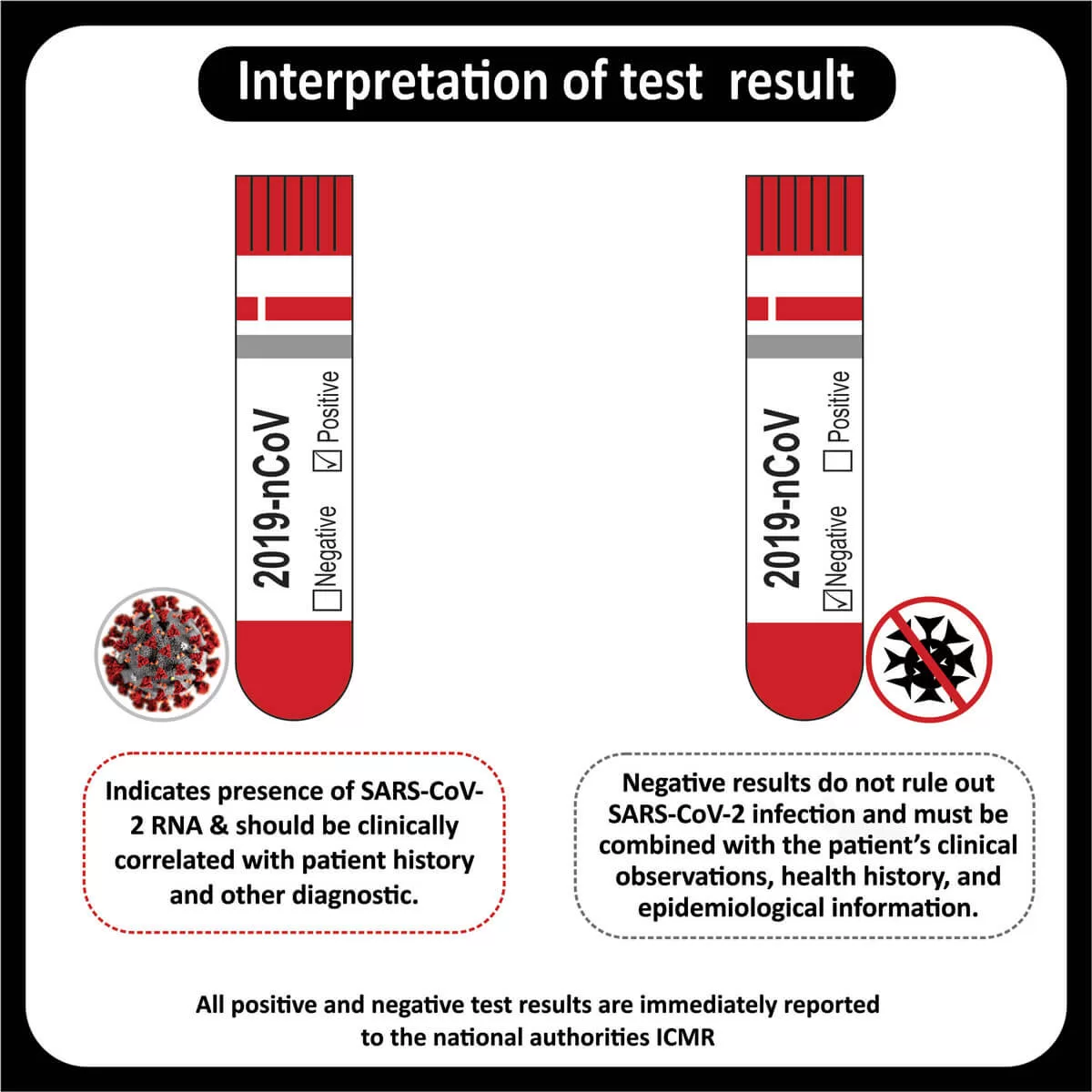
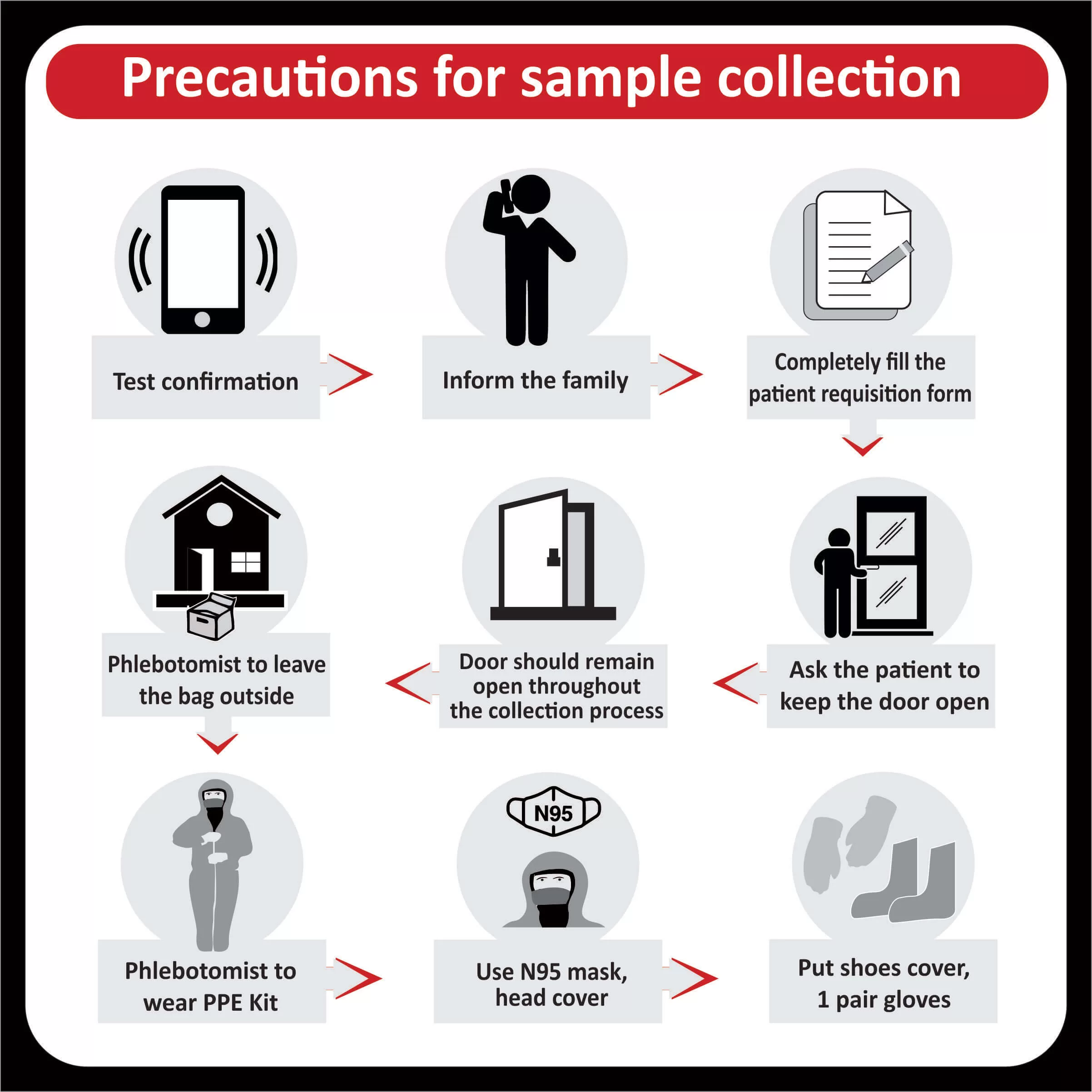
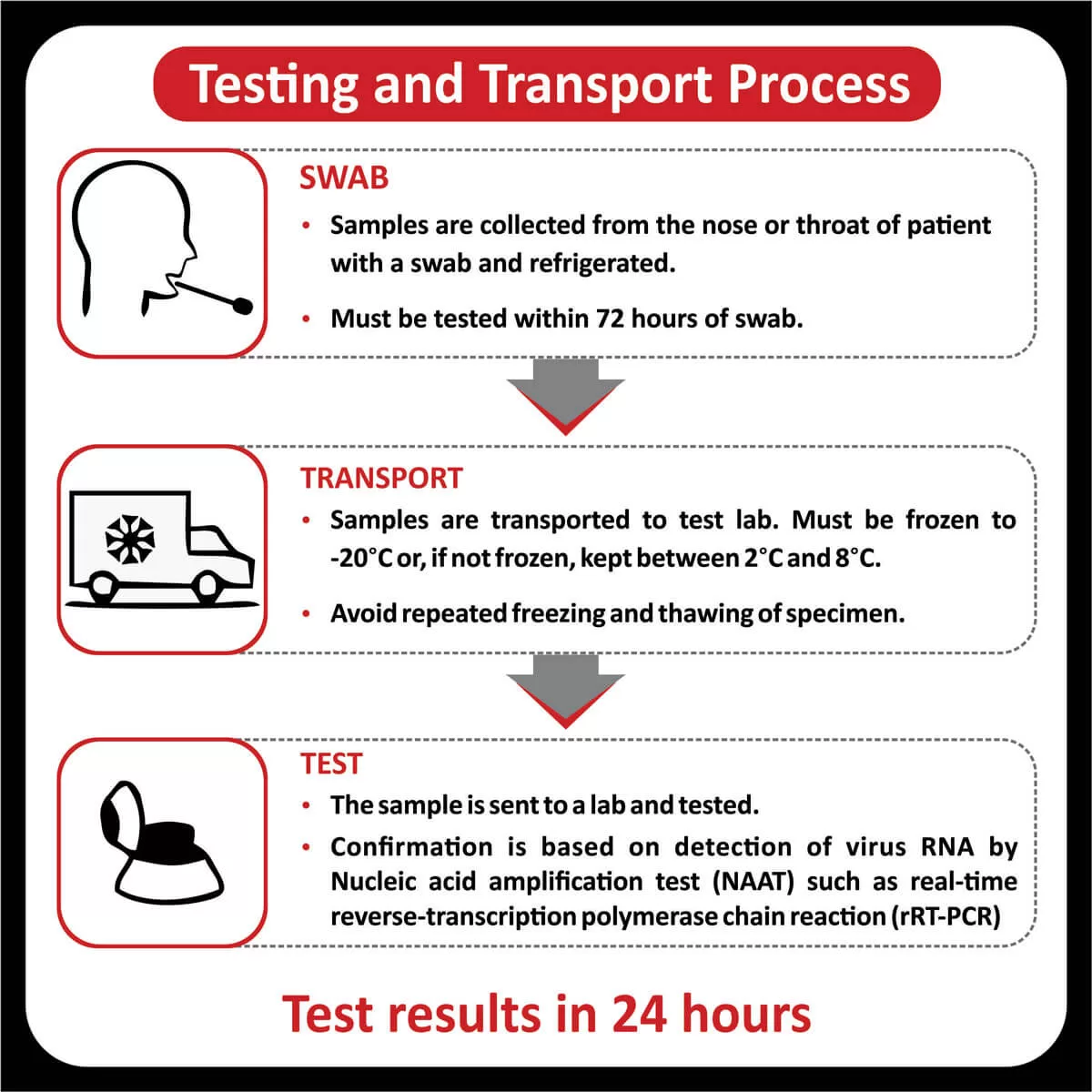
The US Food and Drug Administration (FDA) has approved Pembrolizumab (Keytruda) for patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy whose tumors express PD-L1 (CPS ≥1) (Read more). The FDA has also approved the expanded use of the PD-L1 IHC 22C3 pharmDx IVD assay as a companion diagnostic test for Keytruda (Pembrolizumab) for cervical cancer (Read more).
In case of gastric cancers, Pembrolizumab did not significantly improve overall survival compared with paclitaxel as second-line therapy with PD-L1 CPS of 1 or higher (Read more). Analysis favored paclitaxel for first 8 months whereas Pembrolizumab showed benefit and prolonged stable disease after 8 months.
Recent clinical trials revealed, Brigatinib has shown substantial intra-cranial response and good progression-free survival in ALK-positive NSCLC and brain metastases patients pre-treated with Crizotinib (Read more).
Ivosidenib, an oral small-molecule inhibitor of mutant IDH1 has shown complete remission in 21.6% and complete remission with partial hematologic recovery in 30.4% IDH1 mutated relapsed or refractory acute myeloid leukemia patients (Read more).
Results from recent clinical trials have shown that addition of Sprycel with intensive chemotherapy has produced good long-term outcomes in patients newly diagnosed with Philadelphia chromosome (Ph)-positive acute lymphoblastic leukemia (ALL). Outcomes were found to be poorer in patients having IKZF1 deletions (Read more).
The US Food and Drug Administration (FDA) has approved a combination of Encorafenib (Braftovi) and Binimetinib (Mektovi) for patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, as detected by an FDA-approved test (Read more).