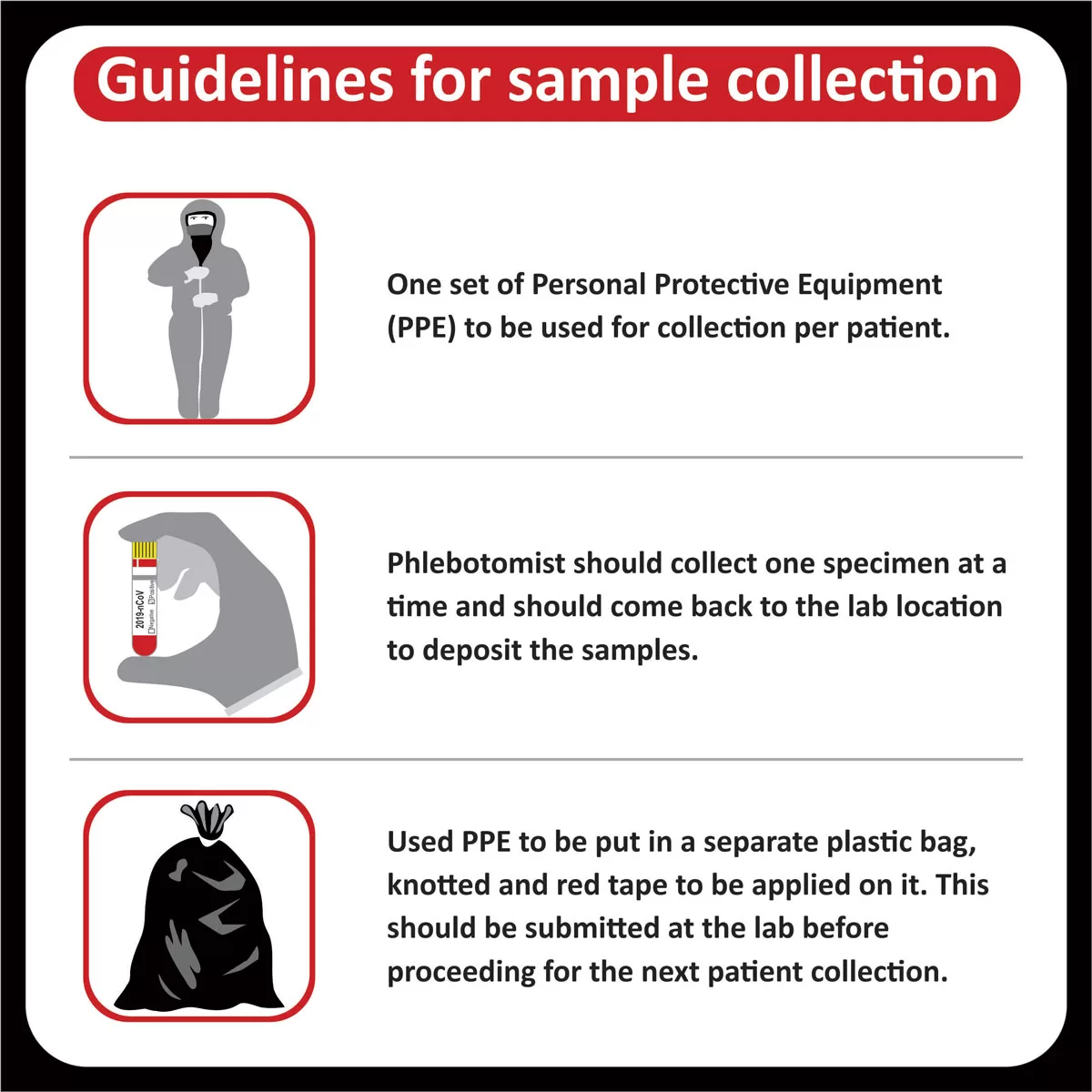
- Case Number :
- Patient Name :
- Age/Sex :
- Patient Location :
- Hospital Name :
- Physician Name :
- Date & Time of Accessioning :
- Date & Time of Reporting :
TEST NAME
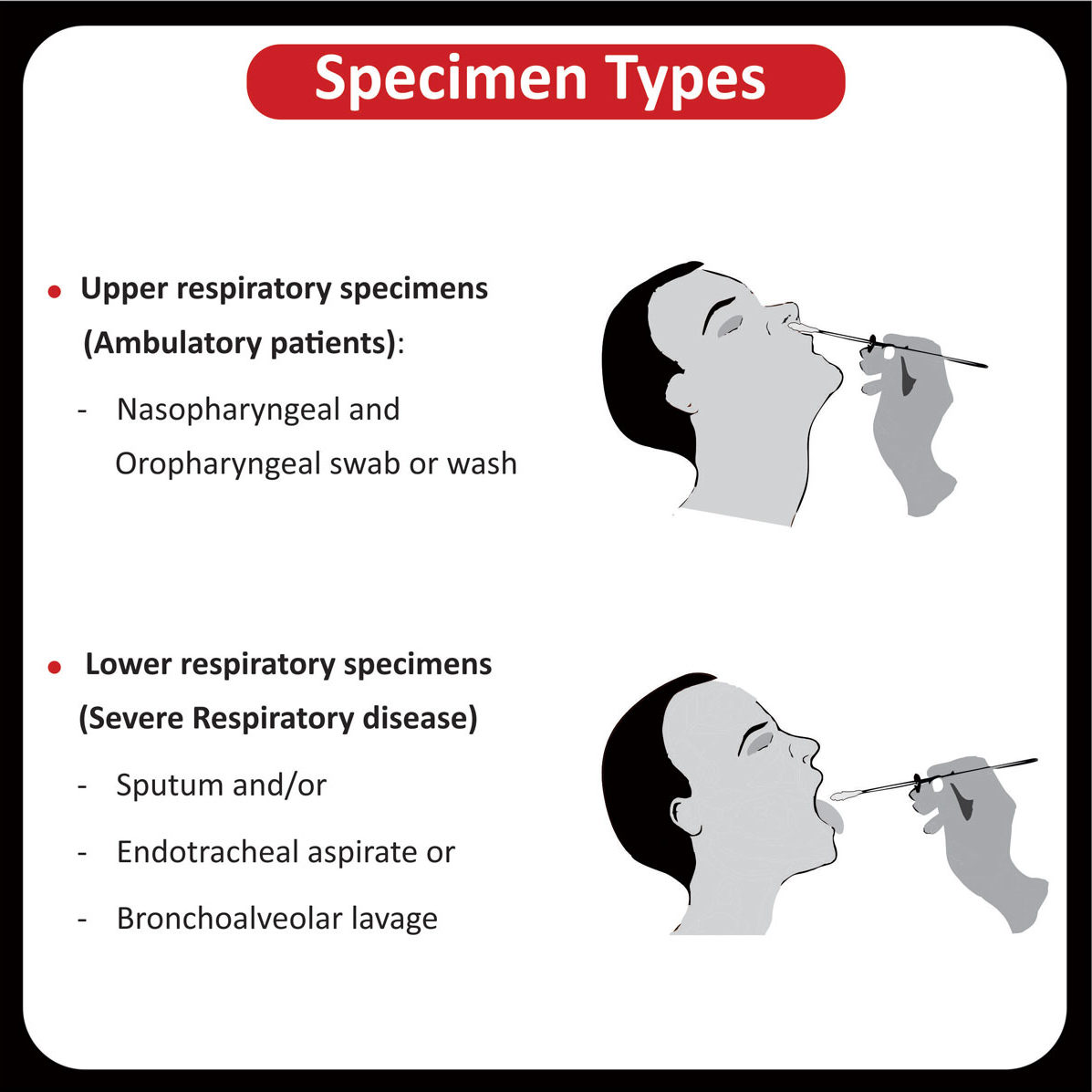
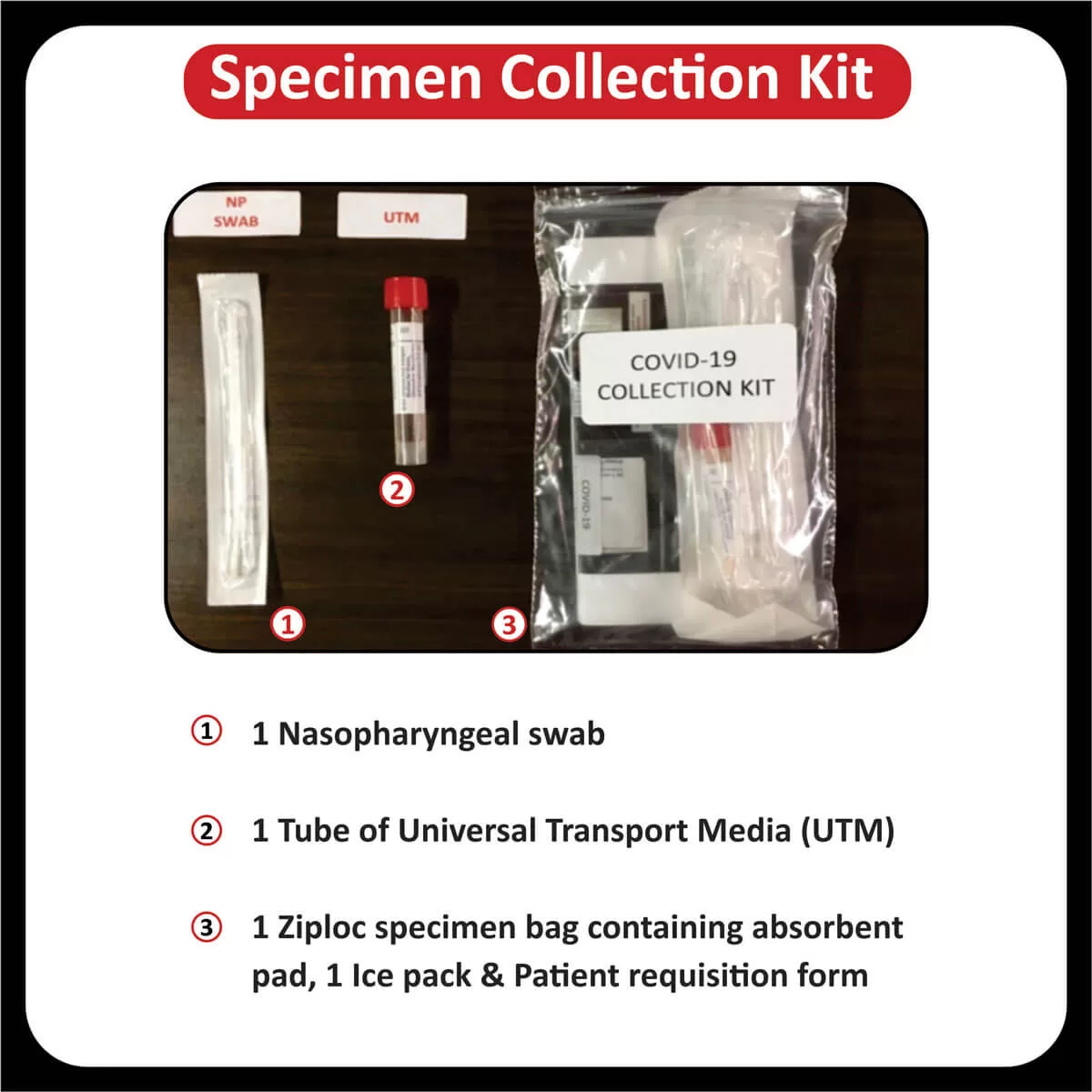
SPECIMEN INFORMATION
CLINICAL HISTORY
METHODOLOGY
Final Diagnosis
- Integrated diagnosis: Diffuse glioma,H3K27 mutant , WHO grade IV
- Histologic diagnosis : Diffuse astrocytoma , WHO grade II of IV , IDH-1 negative by IHC
COMMENT Comments from the pathologists on their approach to the rendered diagnosis with further recommendations for molecular/ cytogenetic studies
Specimen consists of biopsy fragments of while matter with a moderately atypical infiltrate of astrocytic cells. there is no evidence of vascular proliferation or necrosis. Scattered mitosis is seen.
Although histologic grade is that of a grade II astrocytoma the positivity for H3K27 M is consistent with a diagnosis of a diffuse midline glioma, H3K27 M mutant which are considered as grade IV lesions due to their historically aggressive clinical behaviour.
Recommendation
- Microscopic and immunohistochemically assessments
- Detailed clinico-pathologic correlation predicting the response and guiding the treatment.
- In specialized branches, like neuropathology, giving an integrated report
Correlation with radiology findings is recommended to confirm midline location of the tumor
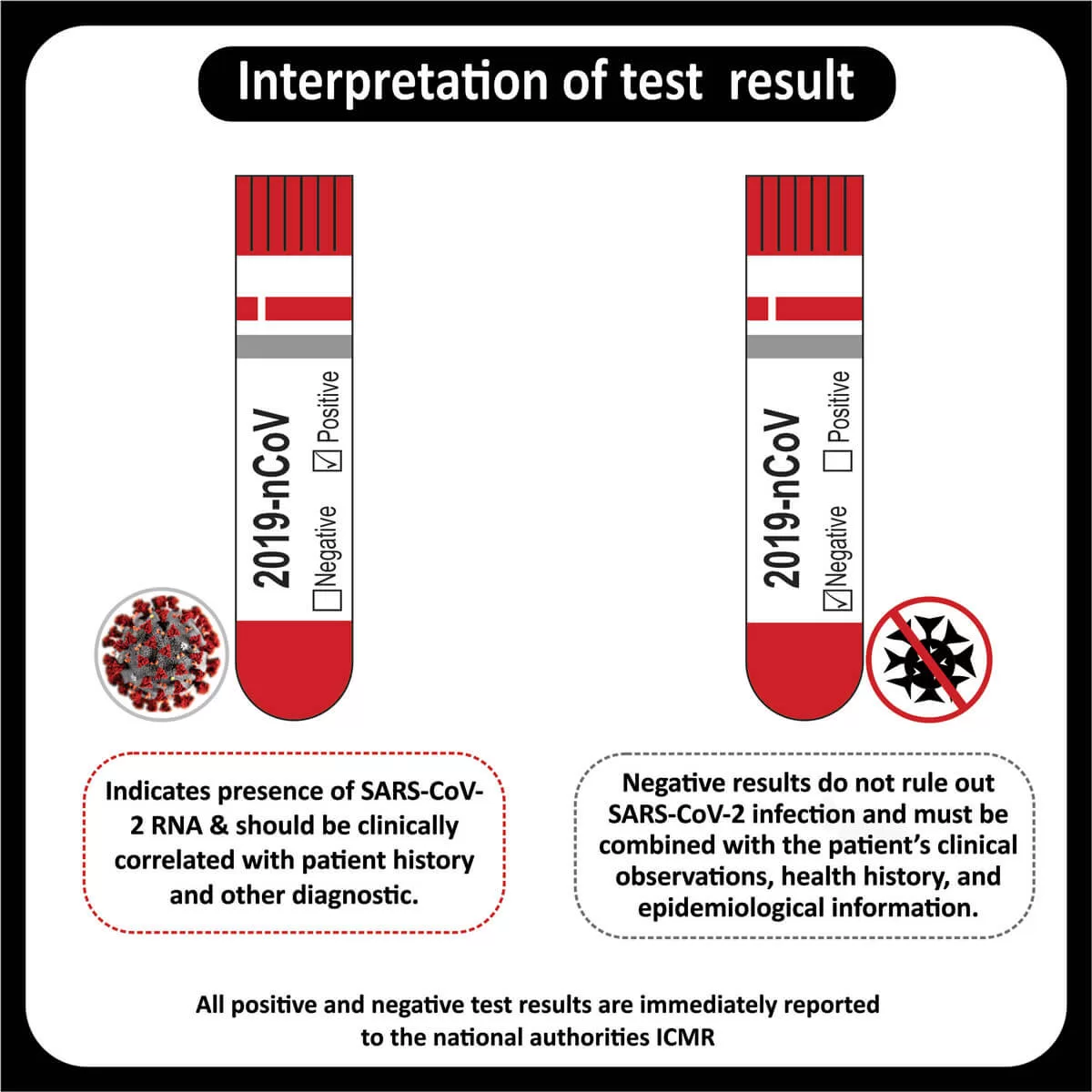
Immunohistochemistry studies
GFAP : Diffuse positive
IDH : Negative (R132 H IHC),
ATRX : Lost
P53: Negative(wild type),
H3K27 M : Positive(consistent with mutant)
Ki67 5 -7%,

Page 1 of 3
- Case Number :
- Patient Name :
- Age/Sex :
- Patient Location :
- Hospital Name :
- Physician Name :
- Date & Time of Accessioning :
- Date & Time of Reporting :
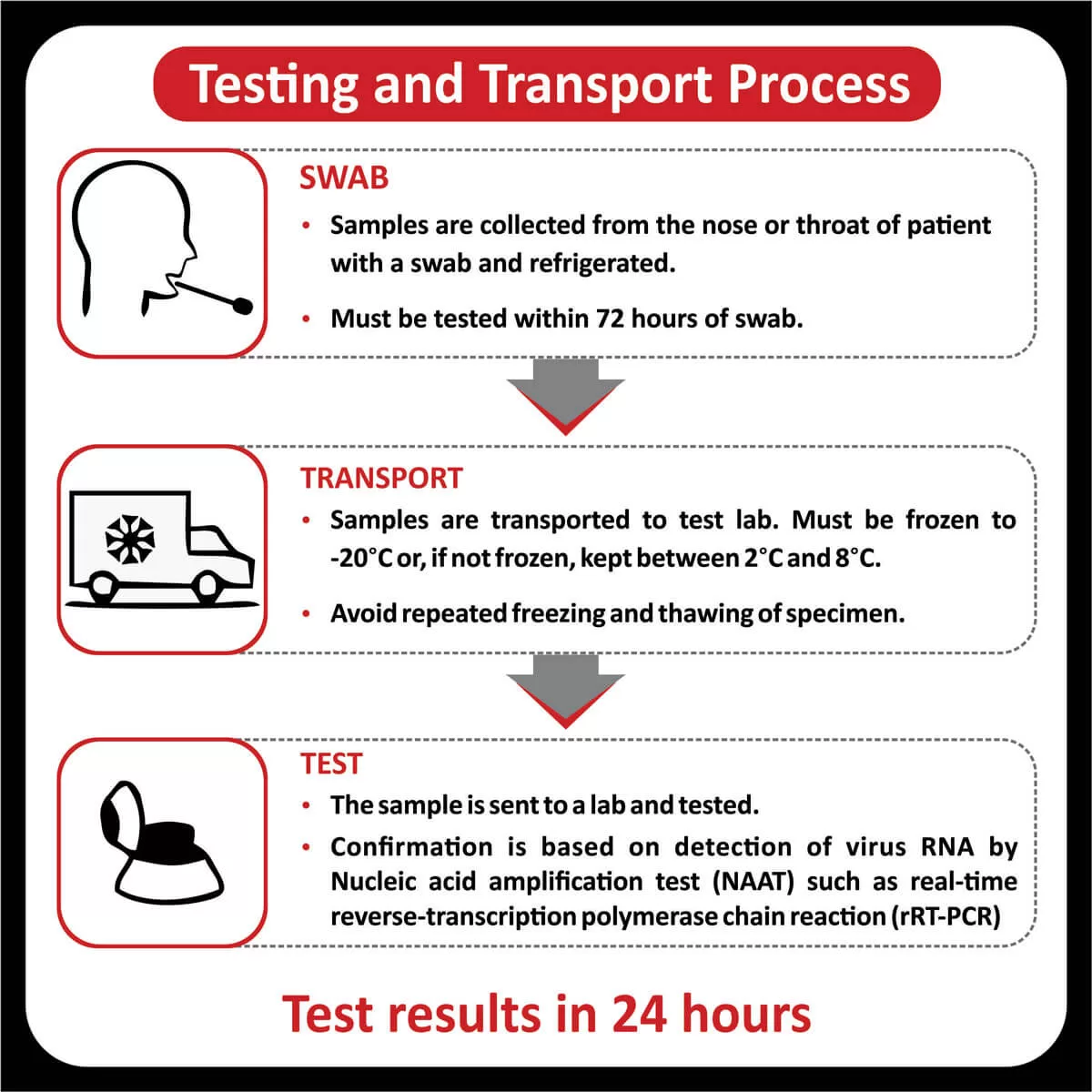
IMMUNOHISTOCHEMISTRY STUDIESIHC images for the better view of the positive/ relevant markers
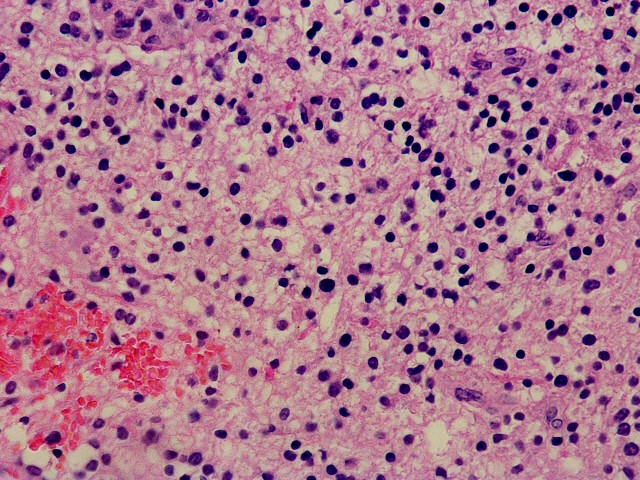
H&E
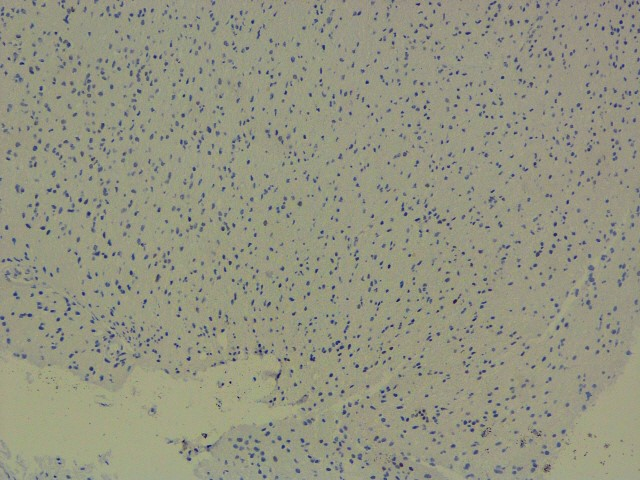
P53 (BP 53-12)
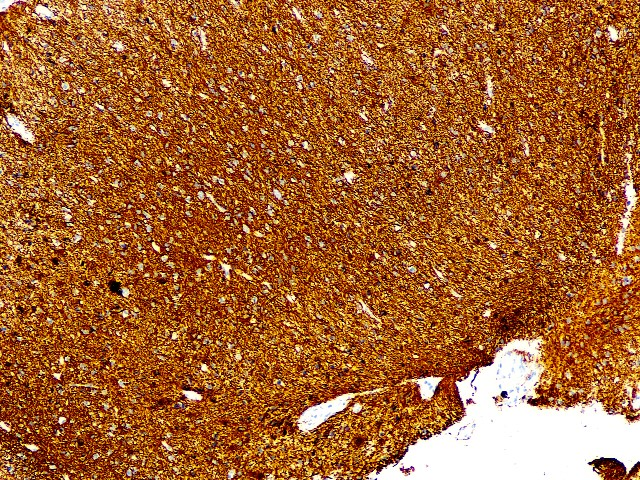
GFAP (Polyclonal)
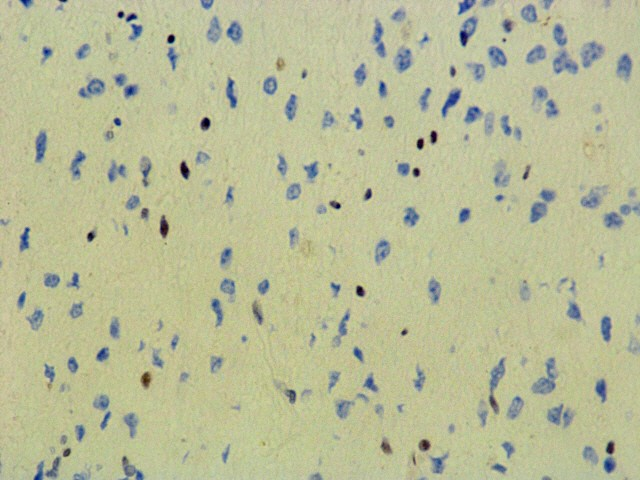
ATRX (D-5)
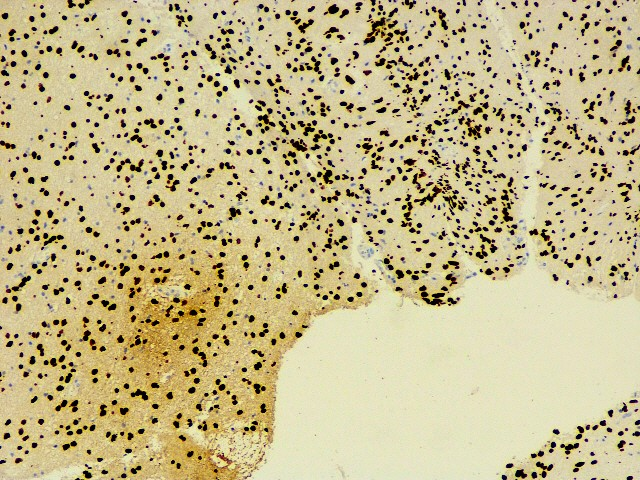
H3K27 M.
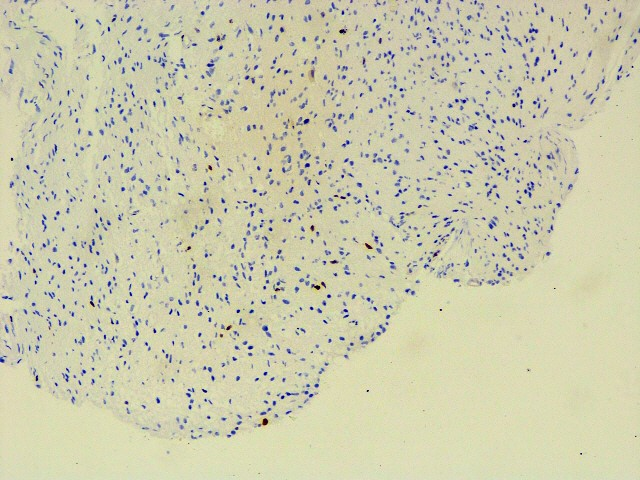
Ki67 (MIB-1)
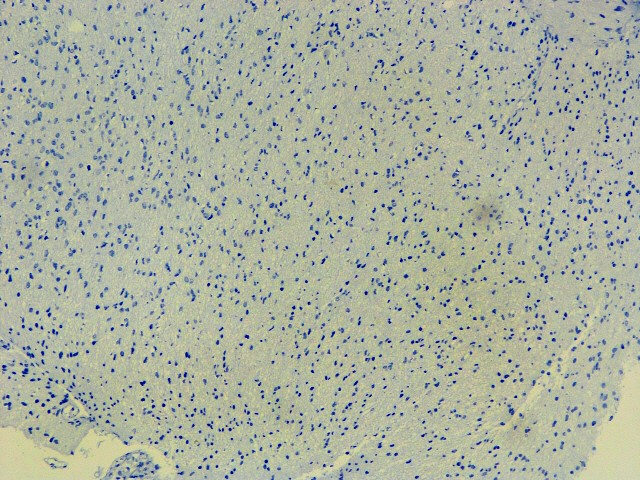
IDH-1 (R132 H)
TECHNICAL NOTE
These assays have not been validated on decalcified specimens.

Page 2 of 3
If you have any questions about this report or would like to have a conversation about the test results,please feel free to reach out to us at
CONDITIONS OF REPORTING
- The tests are carried out in the lab with the presumption that the specimen belongs to the patient named or identified in the bill/test request form.
- The test results relate specifically to the sample received in the lab and are presumed to have been generated and transported per specific instructions given by the physicians/laboratory.
- The reported results are for information and are subject to confirmation and interpretation by the referring doctor.
- Some tests are referred to other laboratories to provide a wider test menu to the customer.
- CORE Diagnostics Pvt. Ltd. shall in no event be liable for accidental damage, loss, or destruction of specimen,which is not attributable to any direct and mala fide act or omission of CORE Diagnostics Pvt. Ltd. or its employees. Liability of CORE Diagnostics Pvt. Ltd. for deficiency of services, or other errors and omissions shall be limited to fee paid by the patient for the relevant laboratory services.