ibs-smart®
|
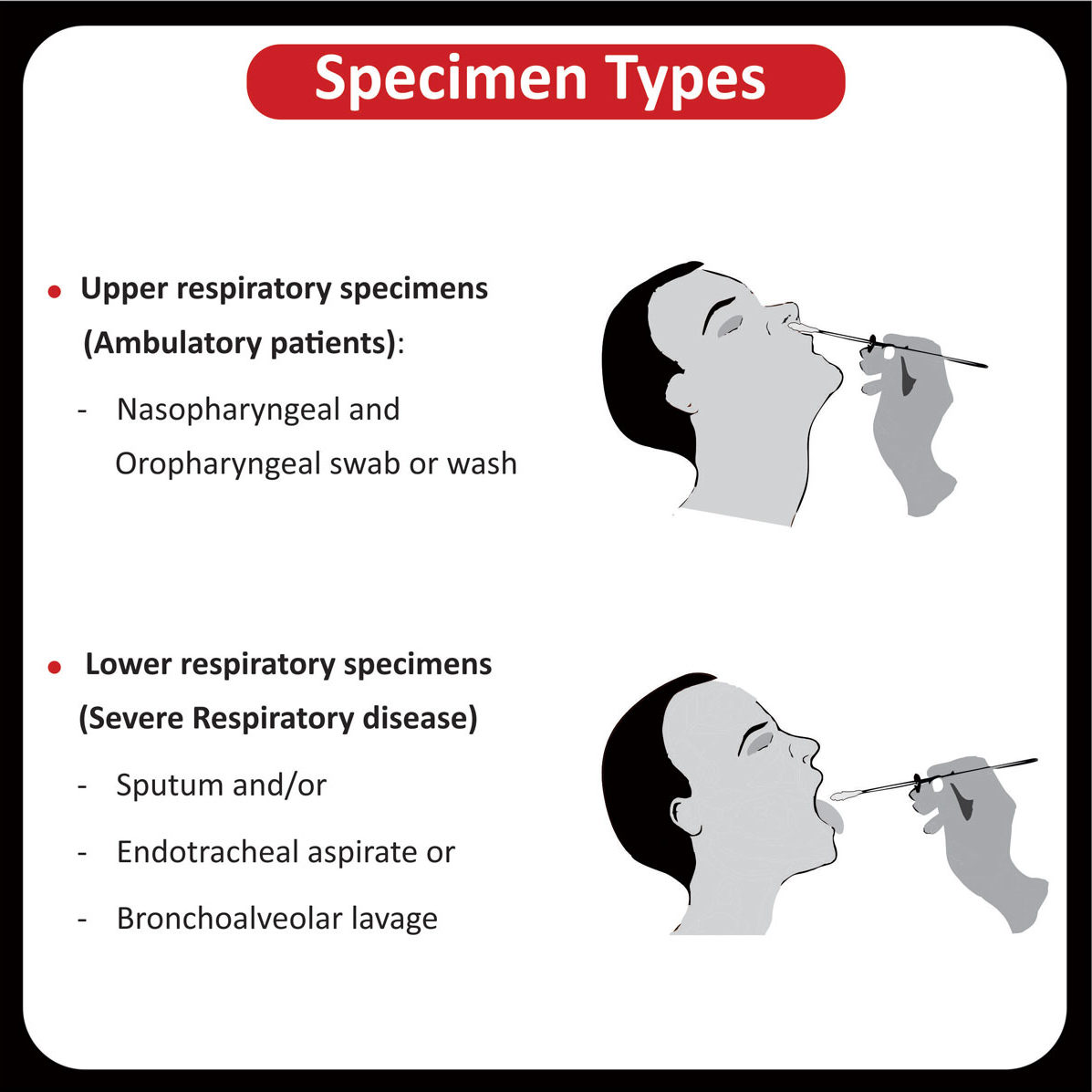
Specimen Type |
|
| EDTA Blood | |
|
Specimen Collection Date & Time |
Date & Time of Accessioning |
- Case Number :
- Patient Name :
- Age/Sex :
- DOB :
- Patient Location :
- Hospital Name :
- Physician Name :
TEST INFORMATION
gastriCORE ibs‐smart® test is a second‐generation biomarker test that measures the level of two antibodies, anti‐Cdtb and anti‐vinculin which are elevated in a majority of patients who have IBS with diarrheal component (IBS‐D or IBS‐M). This test is highly specific in distinguishing IBS from IBD.
CLINICAL HISTORY
METHODOLOGY
Second generation biomarker – Enzyme Linked Immunosorbent Assay
-
RESULTIf either antibody is “Elevated,” the test results can be considered positive and the patient can be confidently diagnosed with IBS-D or IBS-M.
If both antibodies are “Not Elevated,” the test results can be considered non-indicative of post-infectious IBS. Further testing may be required in this case.Antibody Detected Patient Value (OD) Antibody Levels Anti‐CdtB Ab 2.71 Elevated Anti‐Vinculin Ab 1.61 Elevated
INTERPRETATION
| Antibody | Reference Interval | Reportable Range |
|---|---|---|
| Anti‐CdtB Ab | 0.00 – 1.56 | 0.00 – 4.00 |
| Anti‐Vinculin Ab | 0.00 – 1.60 | 0.00 – 4.00 |
COMMENTS
- ibs‐smart® measures validated biomarkers, anti‐CdtB and anti‐vinculin, used to diagnose irritable bowel syndrome (IBS) with 96%‐100% positive predictive value.
- These biomarkers are elevated in >60% of patients with diarrheal IBS (IBS‐D) and mixed diarrheal/constipation IBS (IBS‐M) indi‐cating the root cause of IBS is likely a lasting microbiome disruption from an instance of gastroenteritis.
- Elevated anti‐CdtB levels indicate an immune response to an instance of gastroenteritis.
- Elevated anti‐vinculin levels indicate an autoimmunity has developed.
- If either antibody is ‘Elevated’, the test results are positive and indicative of IBS.
- If both antibodies are ‘Not Elevated’, the test results are considered non‐indicative of post‐infectious IBS, and further testing may be required to diagnose the patient’s GI symptoms.
Page 1 of 3

ibs-smart®
|
Specimen Type |
|
| EDTA Blood | |
|
Specimen Collection Date & Time |
Date & Time of Accessioning |
- Case Number :
- Patient Name :
- Age/Sex :
- DOB :
- Patient Location :
- Hospital Name :
- Physician Name :
REFERENCES
- Morales, W., Rezaie, A., Barlow, G. et al. Second‐Generation Biomarker Testing for Irritable Bowel Syndrome Using Plasma Anti‐CdtB and Anti‐Vinculin Levels. Dig Dis Sci 64, 2019
- Pimentel M, Morales W, et al. Development & validation of a biomarker for diarrhea predominant irritable bowel syndrome in human subjects. PLoS One, 2015.
Page 2 of 3
If you have any questions about this report or would like to have a conversation about the test results,please feel free to reach out to us at
CONDITIONS OF REPORTING
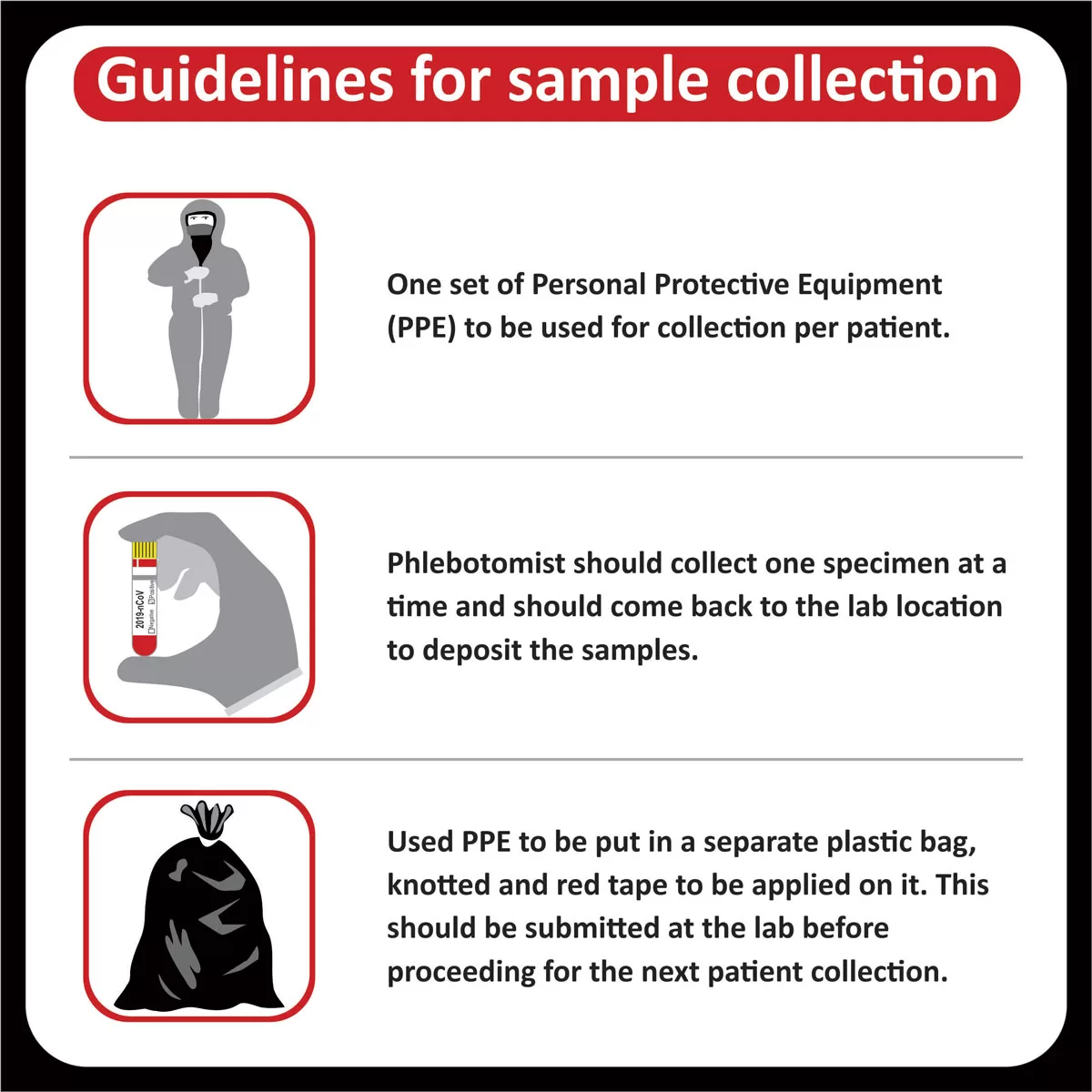
- The tests are carried out in the lab with the presumption that the specimen belongs to the patient named or identified in the bill/test request form.
- The test results relate specifically to the sample received in the lab and are presumed to have been generated and transported per specific instructions given by the physicians/laboratory.
- The reported results are for information and are subject to confirmation and interpretation by the referring doctor.
- Some tests are referred to other laboratories to provide a wider test menu to the customer.
- CORE Diagnostics Pvt. Ltd. shall in no event be liable for accidental damage, loss, or destruction of specimen,which is not attributable to any direct and mala fide act or omission of CORE Diagnostics Pvt. Ltd. or its employees. Liability of CORE Diagnostics Pvt. Ltd. for deficiency of services, or other errors and omissions shall be limited to fee paid by the patient for the relevant laboratory services.
This report is the property of CORE Diagnostics. The information contained in this report is strictly confidential and is only for the use of those authorized. If you have received this report by mistake, please contact CORE Diagnostics
406, Udyog Vihar, Phase III, Gurugram 122016 I 3535 Breakwater Ave., Hayward, CA 94545, USA